LED BY SCIENCE.
DRIVEN BY SERVICE.
Providing State-of-the-Art Pathology Services
OmniPathology is a physician-owned California professional medical corporation. The OmniPathology CLIA certified laboratory is located in the heart of Pasadena. We are specialized in providing academic level, state-of-the-art pathology services. We pride ourselves in our commitment to an excellent standard of service.
PRESS RELEASE
OmniPathology and Delta Labs Announce International Collaboration to Expand Access to Proprietary Oropharyngeal HPV Swab Testing
PASADENA, Calif. | RIYADH, Saudi Arabia (Sept. 15, 2025)—OmniPathology, a U.S.-based advanced molecular diagnostics laboratory, today announced a new international collaboration with Delta Medical Laboratories, a leading clinical laboratory provider in Saudi Arabia.
Under this agreement, Delta Labs will market and offer OmniPathology’s proprietary and patent-pending swab test for oropharyngeal human papillomavirus (HPV) across Saudi Arabia and the broader Middle East region…
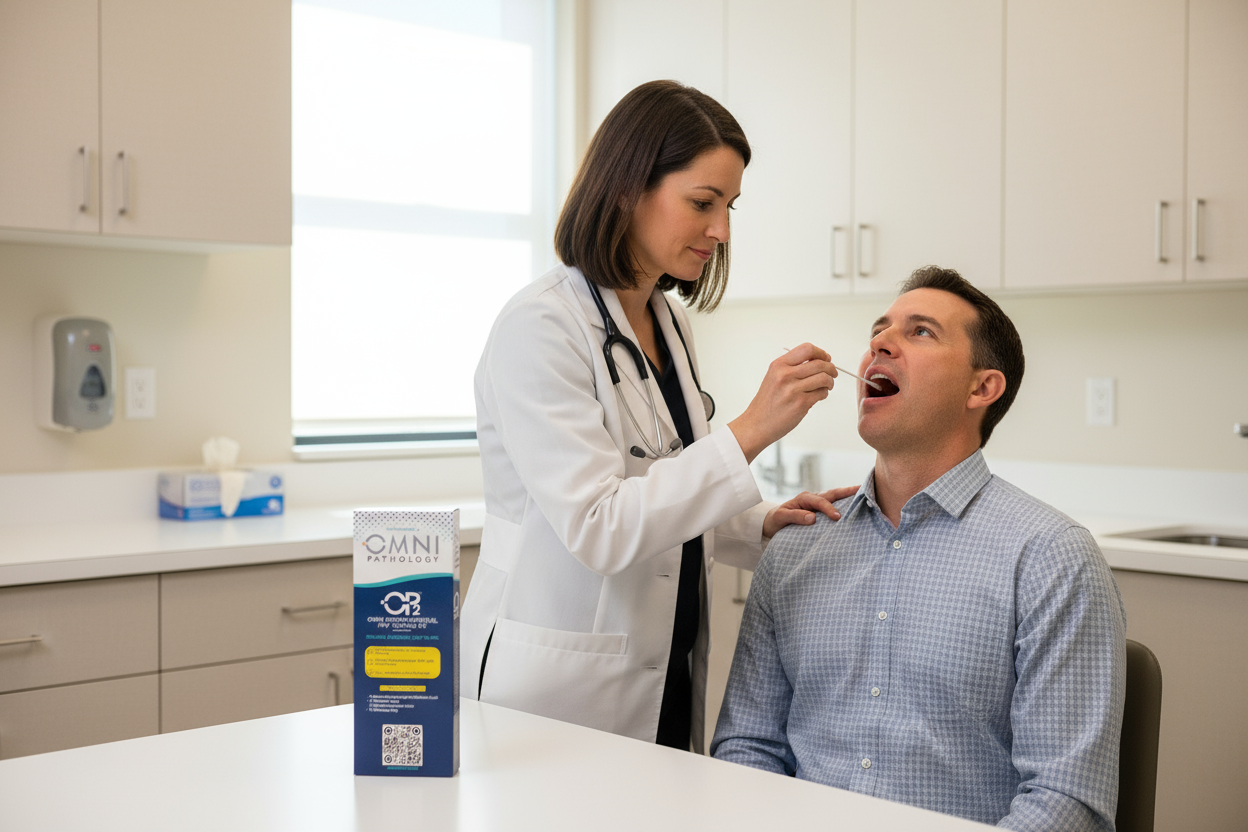
THROAT HPV TESTING WITH OP2™
HPV-related oropharyngeal (throat) cancers are rising. HPV is identified in at least 87% of oropharyngeal cancers.
With a simple throat swab, the OP2™ test can detect high-risk HPV strains early—no blood, no special prep, and fast results through our trusted network of providers or directly at our Pasadena lab.
For Patients
Routine exams give you direct access to the oropharynx—turn that access into early detection. OP2™ integrates into standard visits with no new equipment or complex workflow, delivering reliable, PCR-based results and clear next steps for timely intervention.
For Dentists & Healthcare Providers

PHYSICIANS | LABORATORIES | BIOTECHNOLOGY + MEDICAL DEVICE COMPANIES
Over the years OmniPathology served physicians, laboratories, biotechnology and medical device companies and was able to present a tremendous value that helped our partners to significantly grow their business and improve the quality of their patient care.
Helping Our Partners

Pathological services start with the quality of histology. Our lab has the most advanced histology equipment operated by highly skilled histology technicians, who have years of experience. We perform in-house immunohistochemistry testing providing our pathologists with advanced tools for comprehensive workup of complicated cases.
THE MOST ADVANCED HISTOLOGY EQUIPMENT
Quality of Histology

UNDER THE LEADERSHIP OF DR. MOHAMMAD KAMAL
Under the leadership of Dr. Mohammad Kamal, our pathologists are trained to provide the highest level of personalized service to our physician clients. As part of our quality standard, we have daily group review of all malignant and significant diagnoses in our daily consensus conference.
Highest Level of Personalized Service

-
OmniPathology is a leader in the GI Pathology space. Because of our excellent standards, we have become the number 1 trusted gastrointestinal pathology choice for many the top GI physicians in California. Our team evaluates cases of colon cancer, Barrett’s esophagus and inflammatory bowel disease including Crohn’s disease and ulcerative colitis.
-
Our GYN pathology program is the most comprehensive GYN testing. Our cutting-edge technology covers a robust pap smear technology with automated Thin-Prep slide preparation and computer assisted digital pathology evaluation available to our highly experienced pathologists. Using our advanced fully automated molecular PCR testing platforms, we offer a comprehensive infectious disease menu that covers the most common STI. Our menu includes FDA approved tests for HPV, Chlamydia and Gonorrhea. Our Chlamydia and Gonorrhea tests are also approved on urine for men and women. Our skilled pathologists have extensive experience in evaluating cases of cervical cancer, endometrial cancer.
-
At OmniPathology, our pathologists have experience in evaluating prostate and bladder biopsies to diagnose prostate and bladder cancer respectively. Our immunohistochemistry panels provide the pathologists with tools to work up complicated cases.
We also offer UroVysion, an FDA approved Fluorescent In-Situ Hybridization (FISH) test performed on urine samples to diagnose bladder cancer.